Gene Therapy Development Pipeline
Odylia is continuously evaluating new opportunities to add to our rare disease pipeline. If you have a therapeutic already in development that you believe would be a great match for Odylia, please reach out to us. We can evaluate the potential for integrating your therapeutic or gene target into the Odylia pipeline.
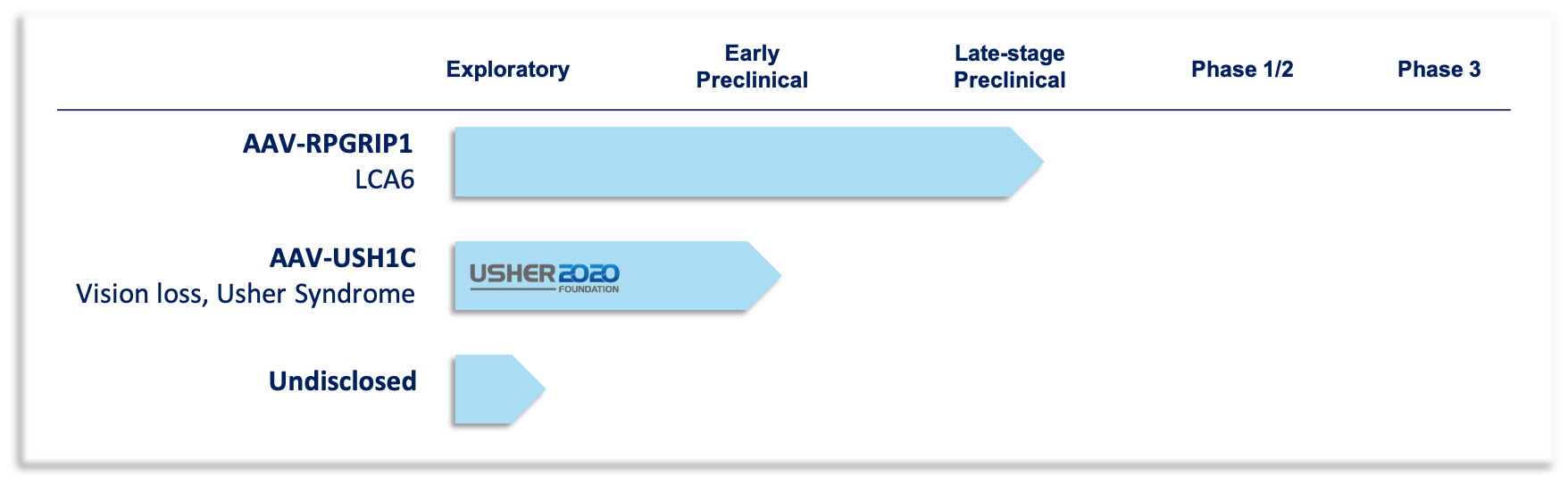
RPGRIP1 Gene Therapy, LCA6
Looking for new partners
Leber congenital amaurosis (LCA) is a rare eye disorder that primarily affects the retina, which is the specialized tissue at the back of the eye that detects light and color. People with this disorder typically have severe visual impairment beginning in infancy. There are currently no treatments for LCA6. Odylia is developing an investigational gene therapy to treat vision loss caused by RPGRIP1 mutations. This gene therapy uses the novel Anc80 vector technology developed by Odylia co-founder, Luk Vandenberghe, and builds upon proof-of-concept data generated at Massachusetts Eye and Ear in the labs of Eric Pierce and Luk Vandenberghe.
USH1C Gene Therapy for the treatment of vision loss, Usher Syndrome Type 1
Partners: Usher 2020 Foundation, FAUN Foundation
Usher Syndrome affects auditory, vestibular, and ocular development. Although rare, this syndrome accounts for roughly 50% of all inherited deaf-blindness disorders. Usher Syndrome can be further broken down into three major types. People with Usher Syndrome type 1 caused by mutations in the USH1C gene are born profoundly deaf, have severe balance problems and begin to lose their eyesight during adolescence. While the auditory deficits can be improved with cochlear implants, there are currently no known treatments for vision loss caused by Usher Syndrome type 1. To overcome this, Odylia has partnered with two patient advocacy groups, the Usher 2020 Foundation and the FAUN Foundation, to develop a novel USH1C gene therapy. Through this partnership Odylia Therapeutics collaborates with scientific experts around the world, including in Germany, the Czech Republic, the United Kingdom, and the United States to streamline development of this gene therapy.



